molecular mass of aluminum|molecular mass of aluminium nitrate : Tagatay Calculate the molar mass of Aluminium in grams per mole or search for a chemical formula or substance. Gabriella Saraivah pelada vazou nudes da buceta e pagando boquete. Assistir porno Gabriella Saraivah nua a Tati de Chiquititas peladinha sexo no xvideos.
0 · molecular mass of aluminum sulfate
1 · molecular mass of aluminium sulphate
2 · molecular mass of aluminium sulfate
3 · molecular mass of aluminium oxide
4 · molecular mass of aluminium nitrate
5 · molecular mass of aluminium hydroxide
6 · molecular mass of aluminium chloride
7 · 1 mole of aluminium
Contrafilé. Exclusivos dos shoppings - Catuaí/ Boulevard/ Est.
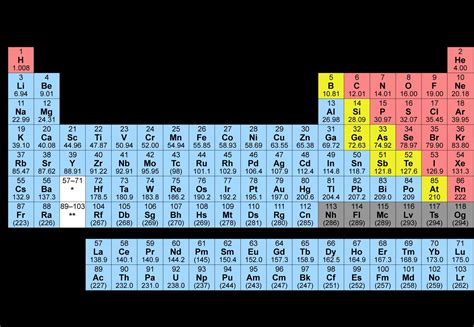
molecular mass of aluminum*******There are 4 easy steps to find the molar mass of Al based on its chemical formula. 1. Count The Number of Each Atom. The first step to finding the molar mass of .Aluminum is the 13th element in the periodic table and has a symbol of Al .Calculate the molar mass of Aluminium in grams per mole or search for a chemical formula or substance.Element Aluminium (Al), Group 13, Atomic Number 13, p-block, Mass 26.982. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.According to the periodic table, the atomic mass of aluminum is 26.98 amu, copper is 63.55 amu, and carbon is 12.01 amu. Since 1 amu is only 1.674 × 10 −24 g, these masses .Formula: Al. Molecular weight: 26.9815386. IUPAC Standard InChI:InChI=1S/Al Copy. IUPAC Standard InChIKey:XAGFODPZIPBFFR-UHFFFAOYSA-N Copy. CAS Registry .
Electron shell. An aluminium atom has 13 electrons, arranged in an electron configuration of [ Ne] 3s2 3p1, [16] with three electrons beyond a stable noble gas configuration. Accordingly, the combined first three ionization .Aluminum is the 13th element in the periodic table and has a symbol of Al and atomic number of 13. It has an atomic weight of 26.98154 and a mass number of 27. Aluminum .Atomic Mass: 26.981539 amu. Melting Point: 660.37 °C (933.52 K, 1220.666 °F) Boiling Point: 2467.0 °C (2740.15 K, 4472.6 °F) Number of Protons/Electrons: 13. Number of Neutrons: 14. Classification: Other . Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.The chemical symbol for Hydrogen is H. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the .Aluminum. Formula: Al. Molecular weight: 26.9815386. IUPAC Standard InChI:InChI=1S/Al Copy. IUPAC Standard InChIKey:XAGFODPZIPBFFR-UHFFFAOYSA-N Copy. CAS Registry Number: 7429-90-5. Chemical structure: This structure is also available as a 2d Mol file. Other names: Aluminium. Permanent link for this species.
26.981. Total Mass. 26.981. The molar mass of Al (Aluminium) is: 26.981 grams/mol. See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the problem set).Q. Find the molar mass oh aluminium oxide. Q. Calculate the number of moles present in 54 grams of aluminium sample. (Molar mass of aluminium is 27). Q. calculate the no.of KJ of heat necessory to raaise the temp. of 60g of aluminium from 35 C TO 55 C MOLAR HEAT CAPACITY OF ALUMINIUM IS 24J/MOL./K MOLAR MASS OF aluminium is .What is the mass of each quantity? 1 mol of Al atoms; 2 mol of U atoms; SOLUTION. One mole of Al atoms has a mass in grams that is numerically equivalent to the atomic mass of aluminum. The periodic table shows that the atomic mass (rounded to two decimal points) of Al is 26.98, so 1 mol of Al atoms has a mass of 26.98 g.molecular mass of aluminum molecular mass of aluminium nitrate Aluminum, chemical element, a lightweight silvery white metal of Group 13 of the periodic table. Aluminum is the most abundant metallic element in Earth’s crust and the most widely used nonferrous metal. . atomic weight: 26.9815384: melting point: 660 °C (1,220 °F) boiling point: 2,467 °C (4,473 °F) specific gravity: 2.70 (at 20 °C [68 . Name: Aluminum (Al). Atomic weight: 26,98. Molecular mass: 26,981539 amu. Number of atoms: Aluminum contains only one atom, which consists of a positively charged nucleus with 13 protons, 14 neutrons inside, and three electron shells with 13 electrons. Melting point: 660 °C. Boiling point: 2518,82 °C. Molar volume: 10,0 cm³/mol.
molecular mass of aluminumAluminum. Formula: Al. Molecular weight: 26.9815386. IUPAC Standard InChI:InChI=1S/Al Copy. IUPAC Standard InChIKey:XAGFODPZIPBFFR-UHFFFAOYSA-N Copy. CAS Registry Number: 7429-90-5. Chemical structure: This structure is also available as a 2d Mol file. Other names: Aluminium. Permanent link for this species. Count the number of atoms of each element in the compound. Find the molar mass of glucose by multiplying the atomic masses of the atoms and their number, then find the sum: μ = 6 × 12.01 g/mol + 12 × 1.0079 g/mol + 6 × 16 g/mol = 180.1548 g/mol. If you know how to calculate molar mass, learn about other ways to express the amount of .molecular mass of aluminium nitrateCalculate the molar mass of Aluminum in grams per mole or search for a chemical formula or substance. . Aluminum molecular weight. Molar mass of Al = 26.981538 g/mol. Convert grams Aluminum to moles. or. moles Aluminum to grams. Percent composition by element. Element: Aluminium Symbol: Al Atomic Mass: 26.981538 # of .
Convert amount of Aluminum in moles to volume and weight using its molecular weight and density. Materials moles to volume and weight calculator. . Aluminum weighs 2.699 gram per cubic centimeter or 2 699 kilogram per cubic meter, i.e. density of aluminum is equal to 2 699 kg/m³; .
A The molecular mass of ethylene glycol can be calculated from its molecular formula using the method illustrated in Example 1: 2C (2 atoms)(12.011 amu/atom) = 24.022 amu . (NO 3) 3 (aluminum nitrate) Cu(ClO 4) 2 [copper(II) perchlorate] Calculate the molecular mass or formula mass of each compound. V 2 O .
Aluminium Hydroxide molecular weight. Molar mass of Al (OH)3 = 78.003558 g/mol. Convert grams Aluminium Hydroxide to moles. or. moles Aluminium Hydroxide to grams. Molecular weight calculation: 26.981538 + (15.9994 + 1.00794)*3.Aluminium Oxide molecular weight. Molar mass of Al2O3 = 101.961276 g/mol. Convert grams Aluminium Oxide to moles. or. moles Aluminium Oxide to grams. Molecular weight calculation: 26.981538*2 + 15.9994*3.

Convert amount of Aluminum in moles to volume and weight using its molecular weight and density. Materials moles to volume and weight calculator. . Aluminum weighs 2.699 gram per cubic centimeter or 2 699 kilogram per cubic meter, i.e. density of aluminum is equal to 2 699 kg/m³; . A The molecular mass of ethylene glycol can be calculated from its molecular formula using the method illustrated in Example 1: 2C (2 atoms)(12.011 amu/atom) = 24.022 amu . (NO 3) 3 (aluminum nitrate) Cu(ClO 4) 2 [copper(II) perchlorate] Calculate the molecular mass or formula mass of each compound. V 2 O .Aluminium Hydroxide molecular weight. Molar mass of Al (OH)3 = 78.003558 g/mol. Convert grams Aluminium Hydroxide to moles. or. moles Aluminium Hydroxide to grams. Molecular weight calculation: 26.981538 + (15.9994 + 1.00794)*3.
Aluminium Oxide molecular weight. Molar mass of Al2O3 = 101.961276 g/mol. Convert grams Aluminium Oxide to moles. or. moles Aluminium Oxide to grams. Molecular weight calculation: 26.981538*2 + 15.9994*3.
1 mol Al = 26.98 g Al. Prepare a concept map and use the proper conversion factor. Cancel units and calculate. 3.987 molAl × 26.98gAl 1 molAl = 107.6gAl 3.987 mol Al × 26.98 g Al 1 mol Al = 107.6 g Al. Think about your result. The calculated value makes sense because it is almost four times times the mass for 1 mole of aluminum.Calculate the molar mass of Aluminium Sulfate in grams per mole or search for a chemical formula or substance. . Aluminium Sulfate molecular weight. Molar mass of Al2(SO4)3 = 342.150876 g/mol. Convert grams Aluminium Sulfate to moles. or. moles Aluminium Sulfate to grams. Molecular weight calculation: 26.981538*2 + (32.065 + .
Aluminum metal is light in weight and silvery-white in appearance. Aluminum is used for beverage cans, pots and pans, airplanes, . Molecular Weight. Property Value. 26.981538 g/mol. Reference. Computed by PubChem 2.2 (PubChem release 2021.10.14) Property Name. Hydrogen Bond Donor Count.Calculate the molar mass of aluminum sulfate in grams per mole or search for a chemical formula or substance. . (SO4)3 = 342.150876 g/mol. Convert grams aluminum sulfate to moles. or. moles aluminum sulfate to grams. Molecular weight calculation: 26.981538*2 + (32.065 + 15.9994*4)*3. Percent composition by element. Element: Aluminium Symbol . Solutions to Example 6.2.2, Summing the molar masses of the atoms in the NaCl formula unit gives. 1 Na molar mass: 22.99 g. 1 Cl molar mass: 35.45 g. Total: 58.44 g. The mass of 1 mol of NaCl is 58.44 g. Multiplying the molar mass of each atom by the number of atoms of that type in bilirubin’s formula and adding the results, we get.3H 2 O), along with other compounds. Karl Joseph Bayer, an Austrian chemist, developed this process in 1888. The Hall-Héroult and Bayer processes are still used today to produce nearly all of the world's aluminum.Examples of molecular weight computations: C[14]O[16]2, S[34]O[16]2. Definitions. Molecular mass (molecular weight) is the mass of one molecule of a substance and is expressed in the unified atomic mass units (u). (1 u is equal to 1/12 the mass of one atom of carbon-12) Molar mass (molar weight) is the mass of one mole of a substance and is .Aluminum nitrate nonahydrate | AlH18N3O18 | CID 24567 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. . Molecular Weight. 375.13 g/mol. Computed by PubChem 2.2 (PubChem release 2021.10.14) Component .
webXEHLI G (@lilwitxehli) no TikTok |31M curtidas.2.8M seguidores.XEHLI G 🩸IG: @xehli.Assista ao último vídeo de XEHLI G (@lilwitxehli).
molecular mass of aluminum|molecular mass of aluminium nitrate